Gossan or the iron cap
Gossan is a term from mineral economics. The gossan may also be called iron cap. This is so because it denotes a concretion of iron hydroxides that has formed on top of sulphide mineral vein, where it reaches the surface. It forms during the supergene sulphide ore enrichment, when weakly acid surface water perloctaes through the mineral deposit. Many sulphide ores are oxidised in this process and brought into solution:
![]()
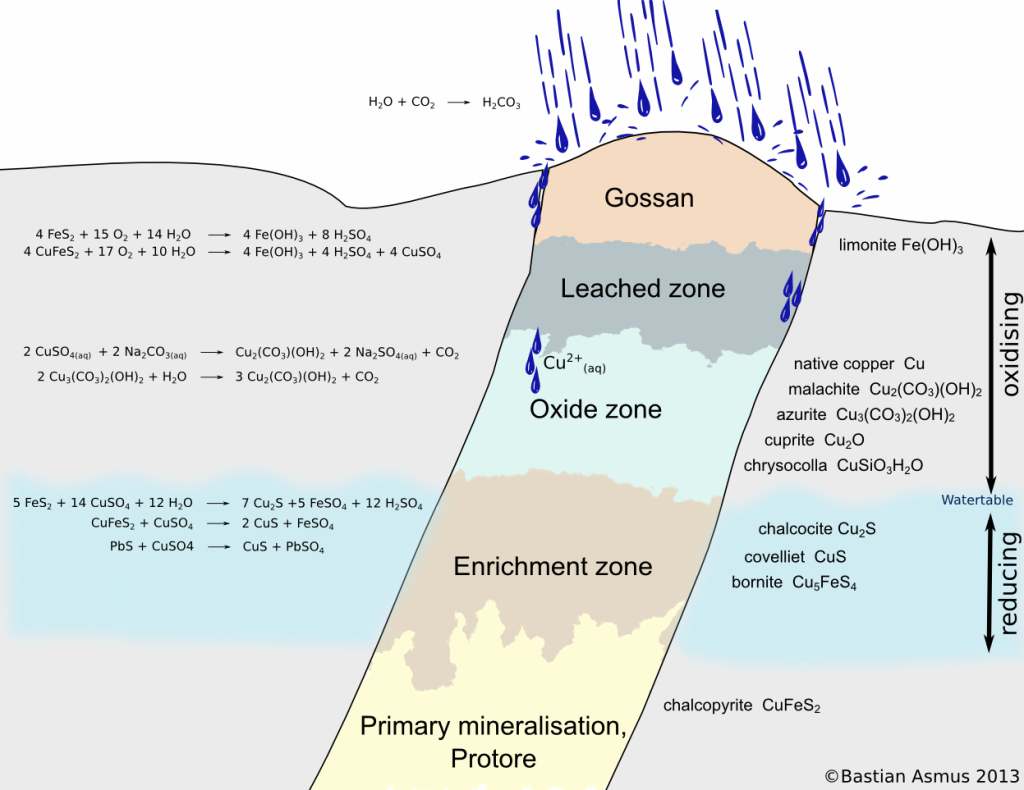
Schematic view of a sulphide vein. You can see the oxidation zone, consisting of the gossan, the leached zone and the oxidised zone. The reducing zone consists of the enrichment zone and the area of primary mineralization. Significantly modified after Evans (1992) and Ottaway (1994).
The resulting solutions may dissolve further minerals (Evans 1992). In sulphide ore bodies for example pyrite (Fe2S) breaks down to sulphuric acid and limonite (Fe(OH)3). Limonite is insoluble in water and remains in the upper zones of the oxidised ore body. Since the formation of limonite is accompanied by an increase in volume, the gossan forms in a particular way, was easily recognized by prospectors and indicated the presence of an ore body to them.
![]()
In the underlying leached oxide zone other ore minerals are dissolved by the sulphuric acid. The ore body is “leached” and the metal ions are transported down to where they may be partly precipitated as oxides again. A zone with oxidized ore remains. Carbonated, oxidizing water may form carbonates such as malachite or azurite (Menschel & Usdowski 1975), eg:
![]()
The copper ions of the dissolved copper sulphate CuSO4 react with carbonates which are also easily dissolved in carbonated water. Malachite Cu2(CO3)(OH)2 or azurite Cu3(CO3)2(OH)2 are thereby precipitated. In contact with water azurite reacts to malachite. However, other ores we cuprite, chrysocolla, or even native copper can occur in this zone.
The greater part of the dissolved metal freight is reprecipitated in the reducing enrichment zone below the water table. Thus the ores of the enrichment zone may significantly surpass the metal content of the primary mineralisation. Typical reactions are:
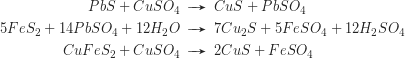
Early archaeological evidence for the use of malachite and native copper
The oxidation zone of a vein mineralisation is also of crucial importance for the emergence of metallurgy, because there you may find native copper and oxidic ores such as malachite side by side. There is evidence as early as 9500 years BC for the intentional collection of copper minerals. In Hallan Cemi and Çayönü Tepesi in Anatolia, we have proof for the first malachite fragments in a settlement context. Only a few hundred years later at 8200 BCE the first objects (mostly beads) made from malachite and copper, appear in Çayönü Tepesi (Yalçin 2000). This was native copper from the oxidation zone of a vein mineralisation that hammered into shape.
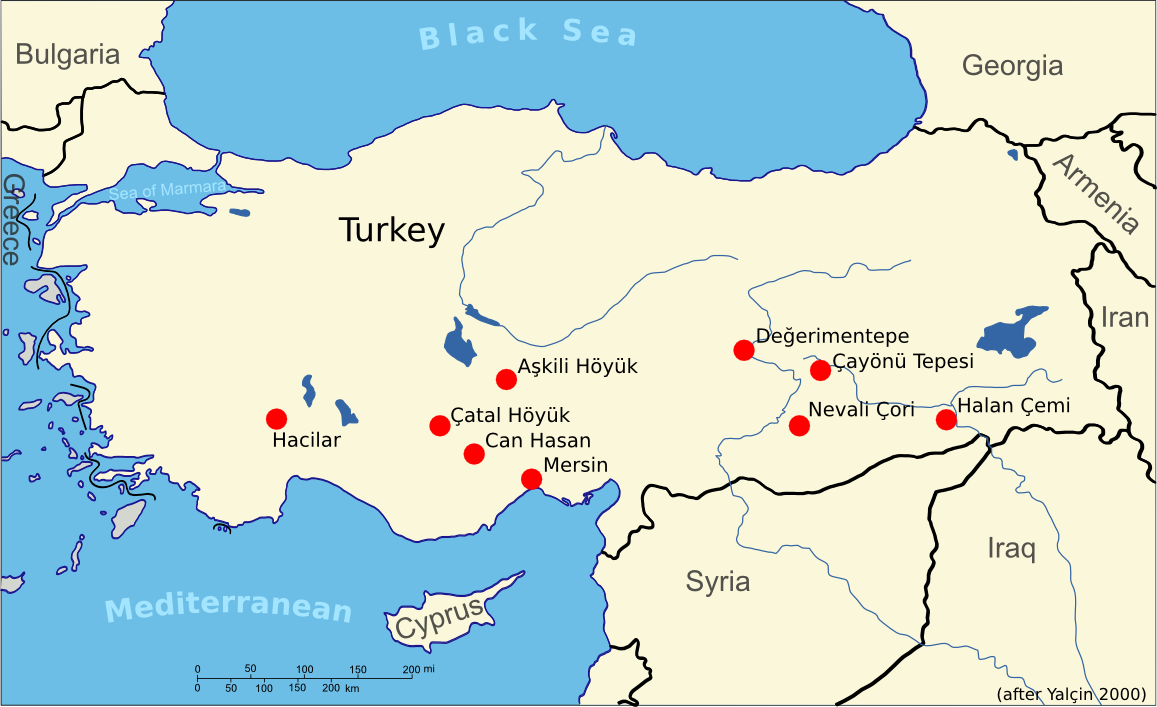
Archaeological settlements with very early metalworking evidence prior to 5000 BC. Data from Yalçin (2000).
References
Evans, A.M., 1992. Erzlagerstättenkunde. Übers. von Udo Neumann und Gerburg Larsen. Enke, Stuttgart.
Ottaway, B.S., 1994. Prähistorische Archäometallurgie. Verlag Marie L. Leidorf, Espelkamp.
Menschel,G. und Usdowski, E. 1975. Experimentelle Untersuchungen über die Stabilität von Cu-Karbonat zur Klärung der Genese von Azurit im Cornberger Sandstein. Contributions to Mineralogy and Petrology 49, 141–147.
Yalçin, Ü., 2000. Anfänge der Metallverwendung in Anatolien, in: Anatolian Metal, Veröffentlichungen aus dem Deutschen Bergbau-Museum Bochum. Bochum.





