Jan
17
2014
Bastian Asmus


This is part three of the series on slag microscopy. Today is about sample mounting: we use a cold mounting procedure, i.e. samples are mounted in resin. I have talked about the find documentation process and the cutting of the sample in parts one and two.

Sample mounting: Three different sizes of sample cups: 20, 32 and 40 mm.
The following things are needed for this:
- cold mounting resin and curing agent
- mixing cup
- sample cups
- ultrasonic bath
- IMS
- optional: exsiccator and vacuum pump
- safety goggles
- lab coat or an apron Continue reading
1 comment | tags: How to | posted in Archaeometallurgy, General, Info, Lab work, Microscopy, slag
Jan
17
2014
Bastian Asmus
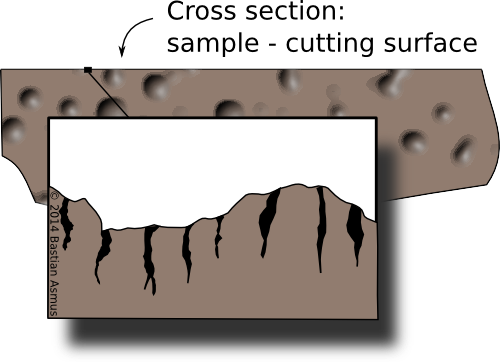
This shows a cross section through a sample. On a micro-scale the cutting surface of a samples is shattered by the cutting process. This areas needs to be removed for samples to be representative of the find.
This is part two of the series on slag microscopy and will deal with sample cutting. Part one was on the macroscopic description and documentation of the sample. Of course these steps may equally employed to mount samples other than slag.
The following things are needed for this:
- a circular diamond blade cutter / tile cutter
- ultrasonic bath
- IMS
- safety goggles
- ear protection
- lab coat or an apron Continue reading
2 comments | tags: How to | posted in Archaeometallurgy, General, Lab work, Microscopy, slag
Jan
15
2014
Bastian Asmus

Image 1: The series on slag microscopy you will tell you about how to prepare, describe and interpret polished sections of slag samples. After that you to get hold of a microscope and invest time…
This is the first article of a series on slag microscopy. Excuse me, on what?
Yes you did read correctly: I said slag microscopy. –
This will be a series of articles thought as an introduction to the most noble and useful art of slag microscopy, the equipment needed to do slag microscopy and what slag microscopy is all about. Today’s topic is sample preparation! Slag microscopy is a small but fun part of one the most fascinating disciplines of archaeology: archaeometallurgy (: and it is very useful for us archaeometallurgists, too.
Why? Because slag tells us a lot about the processes and products of which slag is a by-product. In order to be able to tell what slag tells us we need to have a look at it through the microscope. We have to prepare a polished section and will describe the microscopical appearance of the sample under the polarising reflected light microscope.
1 comment | tags: How to | posted in Archaeometallurgy, comparative collection, Lab work, Microscopy, Science, slag



